Research
From Biosynthesis to Functional Biomaterials
Microbes and plants synthesize a tremendous diversity of chemical compounds that is unmatched by chemical synthetic methods. Biological systems use enzymes to achieve the synthesis of complex molecules at room temperature and in the absence of organic solvents. We are exploring and utilizing these enzymatic machineries to enable the sustainable production of valuable chemicals in recombinant microbes and in cell-free biocatalytic systems.
Self-organization at the nano- to macro scale is a key characteristic of all biological systems. Inspired by natures ingenuity and the prospects offered by biomolecular self-assembly, we are characterizing and designing genetically programmable protein-based nanoarchitectures for a wide range of applications in biocatalysis, biosynthesis, and for the fabrication of new types of bio(inorganic)-nanomaterials and living materials.
Biological Self-organization
Self-assembling protein nanomaterials
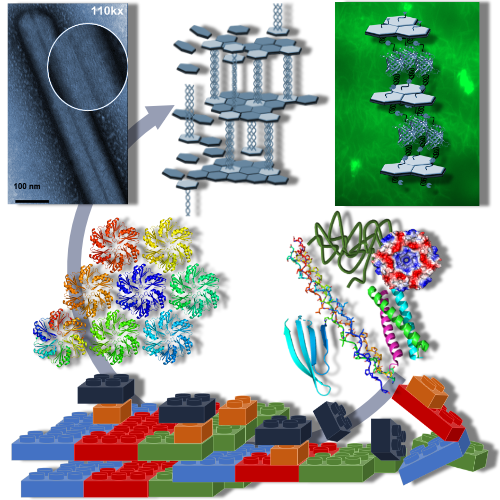
In biological systems, proteins, nucleic acids and lipids are precisely organized to form higher ordered structures. Principles underlying the assembly and organization of natural bionanomaterials can be harnessed for the design and fabrication of functional biomaterials across multiple scales for different applications.
Self-assembly also provides a low-cost approach for the bottom-up construction of supramolecular biomaterials from simple building blocks. By varying building block composition, molecular structures and environmental conditions, assemblies can be controlled and altered by changing interaction types and strengths.
Peptides and proteins offer the most versatility for the assembly of such designed structures due to the chemical diversity of their amino acid components. Unlike nucleic acid-based structures, protein materials are also highly robust and can be readily modified and tailored for desired applications. Importantly, materials built from protein building blocks are genetically encoded. As such, they are programmable and can be produced recombinantly at low cost.
We are designing protein-based nanomaterials from recombinantly produced building blocks. In previous work, we have designed and characterized a toolbox of hexameric proteins that assemble into ultra-robust and easy to engineer scaffolds. Current efforts are aimed at expanding this platform for the design and macroscale formation of hierarchical 3D assemblies for a range of applications
Biomaterials & Living Materials
Biomineralization and Biocomposites
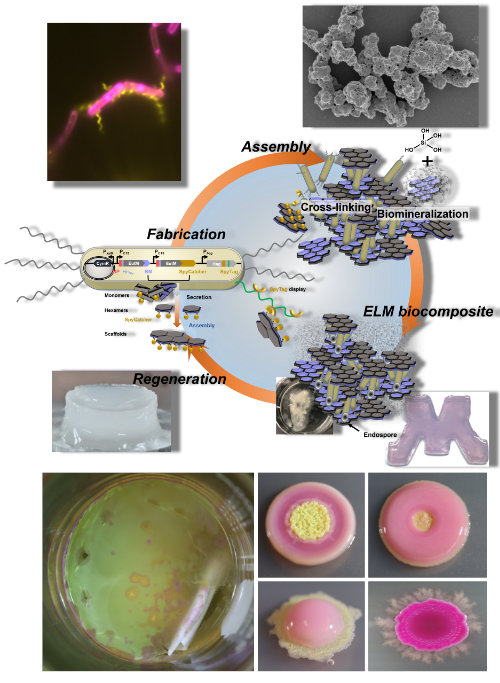
Living systems produce a multitude of hierarchical organic-inorganic composite materials with properties and functions unmatched by synthetic materials. Unlike man-made materials that are fabricated according to a fixed design, biogenic materials are grown and produced from the bottom-up with simple building blocks such as proteins and carbohydrates that create functional surfaces and scaffolds for material formation. Molecular interactions drive the biomineralization of structures that span multiple length scales – from the nanoscale to macroscopic structures such as those of bones or the shells of mollusks and the silica structures made by sponges and diatoms. Inspired by strategies employed by biological systems, a variety of enzyme co-localization and compartmentalization mechanisms are currently explored to optimize multi-enzyme biocatalysis.
Many of these materials have extraordinary properties, which would be of enormous interest for the fabrication of bioinspired materials. For example, biogenic materials combine light weight with light refraction or toughness with strength and elasticity. In addition, natural materials possess the ability to adapt, remodel and self-repair due to their fabrication by living cells and tissues. Learning and then adopting the key principles that underly these processes would enable the design and biofabrication of hierarchical biocomposites – opening the path to materials with superior mechanical properties and functions.
We are investigating natural biomineralization mechanisms and interface them with protein-based nanomaterials for the design of novel types of biocomposites and living materials.
Biofilms
Biofilms are mixed-species conosrtia of micricrobial cells that are organized into hierarchical structures to create microenvironments that confer survival advantages through distributed metabolic functions to access resources, and by providing resistance to environmental stresses. Naturally formed biofilms are readily used in wastewater treatment processes such as for denitrification. We believe that use-inspired biofilm consortia can be designed as robust, multi-functional, high cell density structures for diverse applications ranging from bioremediation, biomanufacturing, energy conversion or as functional surface coatings.
We are investigating design principles to establish a foundational knowledge based that will enable us to engineer, fabricate and deploy use-inspired biofilms for bioremediation and resource recovery applications.
Biomanufacturing
Bioinspired strategies for multi-enzyme catalysis
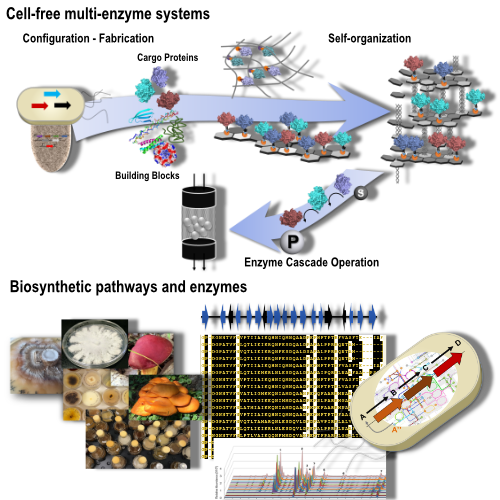
In vitro multi-enzyme catalysis has emerged as a powerful approach for the synthesis of complex chemicals. Yet, the operation of multiple enzymes simultaneously with concurrent recycling of required cofactors can be challenging and requires the development of new strategies for increasing operational performance of enzymatic cascade reactions. Inspired by strategies employed by biological systems, a variety of enzyme co-localization and compartmentalization mechanisms are currently explored to optimize multi-enzyme biocatalysis.
We are leveraging our self-assembling protein nanomaterials for the self-organization and co-immobilization of enzymatic cascade reaction. Our goal is the design of genetically encoded co-localization mechanisms that can become platforms for autonomously self-organizing biocatalytic systems. Such genetically programmable systems can either be produced by cell factories or emerging cell free systems.
Biosynthetic pathway and enzyme discovery
Our group has a longstanding interest in the discovery and design of biosynthetic pathways that enable the production of pharmaceutically relevant compounds. In recent years, we have focused on pathway and enzyme discovery in mushrooms. This class of higher fungi (Basidiomycota) has been used for centuries in traditional medicine, and they are known to produce a range of unique, pharmaceutically relevant chemicals. Yet, the biosynthetic pathways that make these molecules are largely unknown. We have pioneered the identification of biosynthetic pathways for one major group of bioactive compounds – the sesquiterpenoids – made by these fungi.
We have created Field-to-Laboratory Biobank of mushrooms for the discovery new bioactive compounds and the discovery of new biocatalysts. Mushrooms have evolved the unique ability to completely degrade recalcitrant lignocellulose. They have become masters of oxidative biocatalysis that is performed by novel and expanded families of many different types of oxidoreductase enzymes. These enzymes are of great utility for the biomanufacturing of chemicals. We are searching our strain collection for new, useful biocatalysts.